All Eyes on EFSA's new 'independence policy'
Brussels, Wednesday 14 December 2011- The European Food Safety Authority (EFSA) has today published a new independence policy, aimed at improving EFSA's independence in delivering scientific opinions on food safety [1]. This new policy, developed following a public consultation and stakeholder workshop in October, will be up for approval by the EFSA Management Board when it meets on Thursday 15 December, in Warsaw.
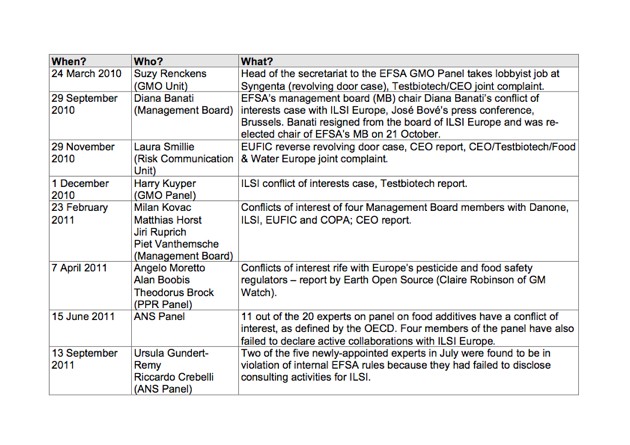
EFSA, which plays a key role in deciding which products and substances are safe to eat, has been under heavy criticism because of apparent conflicts of interest among its staff and experts. Corporate Europe Observatory and other organisations have over documented links between EFSA's management board and some of its expert panels with the agribusiness industry (see table below).
CEO has also criticised EFSA's heavy reliance on industry data and the way in which it systematically excludes independent scientific studies from its assessments.
A draft policy, published by EFSA earlier this year, failed to address many of the conflicts of interest identified, CEO said in response to the consultation.
CEO argues that EFSA has until now failed to recognise the problem posed by conflicts of interest. While the EU institutions are responsible for the membership of EFSA's Management Board, EFSA is responsible for selecting the members of the expert panels, and deciding in which cases there is a conflict of interest.
Nina Holland, campaigner at CEO said:
"Experts who have a vested interest in EFSA decisions because of links to industry should not be allowed to sit on expert panels providing scientific advice. So, for example, someone whose laboratory is funded by Nestlé, should not be allowed on the food additives panel (ANS Panel) as currently is the case, because Nestlé has an active interest in almost all food additives. The new policy must clearly ban conflicts of interest by setting clear criteria for panel members' independence."
CEO will assess ESFA's new independence policy to see whether the radical improvements that are needed have been made.
Last week, the European Ombudsman sided with NGOs, ruling that the EFSA management had failed to act properly in the case of Suzy Renckens, who moved straight through the revolving door into a lobby job with Syngenta after serving on the EFSA GMO Panel.
The European Parliament Environment Committee will discuss EFSA's independence from industry next week, when deciding on the approval of EFSA's 2010 budget (20 December).
For more information please contact:
Nina Holland, Corporate Europe Observatory, nina@corporateeurope.org
Notes:
[1] The Draft Policy on Independence and Scientific Decision-Making Processes incorporating changes resulting from the Public Consultation process will be discussed at the Management Board meeting in Warsaw on Thursday 15 December 2011. The text is available on EFSA website at http://www.efsa.europa.eu/en/mb111215/docs/mb111215-ax8a.pdf
EFSA’s technical report on the outcome of the public consultation on the draft Policy on Independence and Scientific Decision-Making Processes is online at: http://www.efsa.europa.eu/en/supporting/pub/indipendence.htm
Table: Overview of Conflicts of Interest at EFSA (CEO 2011)